
Technology
meets
biology

…thorough and well planned clinical research in humans cannot be replaced, particularly in changing times, despite emerging data science, AI, deep learning and a changing regulatory landscape.
It is our philosophy that any organizational performance is directly related to an organization’s capacity for effective leadership, strategic thinking, organization-wide collaboration, a commitment to service, and shared accountability for results.
Our core competencies and expertise will guide you through the jungle of all changes and areas of pharmaceutical clinical research: regulatory processes, scientific planning, quality management, financial budgeting, implementation, change management, operations and finalization.
Together, we will launch your concepts and clinical research plans, translating visions into successful reality.
The Finom Management Group and its preceeding companies Assign Group and ABF Pharmaceutical Services, had been founded in 2003 and 2005. Since then, we have been deeply involved in the processes and complexities of pharmaceutical drug research and development from a big pharma perspective, from a biotechnologies viewpoint to academic research.
In the world of business,
Warren Buffet
the people who are most successful are those who are doing what they love.
Clinical Development is a costly, legal & ethical journey requiring:
- in-depth knowledge of the legal requirements
- stringent CMC and analytics
- appropriate planning of time tables
- appropriate budgeting und finance planning
- capacity & resource planning
- sufficient and appropriate Quality Assurance
- consideration of change management
- considering anticorruption / money laundering legislation
Challenges to Study Success

Integrity
in all that
we do
Management
The Finom Management Group has over 50 years of experience in drug development. This integrity has been acquired in senior functions in big pharma, biotechnology, academic research and the clinical research service industries. Through the years of operational work, management and leadership, we bring understanding, credibility, real life management practices and a history of success.
We are now a small team of international executives and closely associated companies specializing in the support of pharmaceutical drug development.
This includes all concerned areas in clinical research, business development, insourcing, outsourcing and corporate strategies. We will share our knowledge with you, who will benefit from our expertise. Connecting all leads is the basis for achievement and success, because pharmaceutical clinical research is a complex interaction of different knowledge and different people.

This combination of knowledge and creativity makes us unique: for 20 years, we have been delivering our promise in 35 countries, over 20 therapeutic areas in cooperation with our international network of experts and clinical research organizations.
Client Base by Revenue
History
2010
Establishment of the company, the operations started with the development of proprietary IT applications. Created by experts for experts whose responsibility is to manage and organize clinical research.
2011/2012
First clients started to use FinSystems.
2013
Examples of the use of FinSystems: “FinSys had been introduced in a cloud as “Software as a Service” to cover the business and economic aspects of our business starting 2011. ABF Pharmaceuticals GmbH focuses on complex clinical trial designs and offers a unique combination of logistic services through our global network.
With Finom and the Fin Systems we found a trusted partner for our business, helping us to adapt an IT system to our business needs.”
ABF Pharmaceuticals GmbH is a service provider for complex clinical trial supply and laboratory services and has been using the Fin Systems since 2011 in various areas.
2014
We have been using FinSys in a cloud as “Software as a Service” to cover the commercial business and economic aspects of our business since 2011.
Assign Clinical Research is a midsized CRO company located in Europe. We are always facing changing requirements from our customers to run their project services. FinSys gives us the flexibility to answer our customer’s demands as well as our internal requirements to track documentation status, project results and budgets for numerous projects at the same time.
The fully integrated of ERP and CTMS gives us a major advantage over our competitors in the market. Exact tracking of time spent compared to estimated time required on projects allows us to undertake real time analysis of our business.
History
2014
Time spent for entering data to different IT-Systems is dramatically reduced. All modules are integrated and consequently work on synchronizing data in different systems is no longer needed. This allowed us to free up time to be spent on supporting our client needs.
Since the user interface is common in all parts of the application, our employees find learning new functionality intuitive and therefore internal training costs were reduced to a minimum.
With Finom and the Fin Systems we found a reliable partner for our business, accompanying us into the future of CRA business.”
Assign Clinical Research GmbH has been using the Fin Systems since 2010 in various areas.
2014
Three more Clinical Research Organizations and Pharma staffing companies have been using the Fin Systems since 2014 in various areas in the CRA world of Clinical Research. Starting with the ERP part of the Fin Systems, the scope was extended to CTMS and TMF soon after initial implementation.
2015 through 2017
Continuous use of FinSys and further developments and validation.
2018
FinSys is being used and further developed by new clients.
2020
Onboarding of new clients with strong focus on oncology and ATMPs in cell therapy research.
Focus shifted from technology to research and development.
History

Expertise
We have gained specific experience and knowledge in all phases of clinical development in vaccines, cell therapy, oncology, cancer immunotherapy and personalized medicine.
Experience in Clinical Development /Trials:
- 350 Clinical Trials in total
- 163 Oncology trials
(Phases I – IV)
- 350 Clinical Trials in total
- 163 Oncology trials
(Phases I – IV)
Therapeutic Focus
Oncology
Cancer Immunotherapy
Cell Therapies
Vaccines
Personalized Medicine
This expertise in the pharmaceutical industry was gained on various levels from active work in pharmaceutical development through to running and establishing several companies in life science:
- Specific experience and knowledge in all phases of clinical development
- Design, write and implement quality management systems and SOPs
- Professional management services to our Life Science industry clients.
- Business planning related matters: creating and establishing
- M&A of life science companies
- Budget and control the progress of clinical studies as well as track study specific information.
- Strategic and operational support in defining and implementing effective strategic and operational structures.
- Advising on the involvement of contract research organizations in clinical research
- Implementing Information Technologies (IT) from ERP systems to CTMS to specialized systems, as well as the connection and interfacing of the selected or developed software that manages and controls clinical trials.
Overview by Type of Study/Phase
| Phase | % |
|---|---|
| Medical Devices | 0,5 |
| Non Interventional Study | 2,2 |
| Phase I | 14,2 |
| Phase II | 25,9 |
| Phase III | 48,4 |
| Phase IV/Postmarketing | 8,8 |
| SUM | 100 |
We help you to establish quality management systems which will meet the requirements of the international standard ICH Q10 Pharmaceutical Quality System
Quality Management Systems
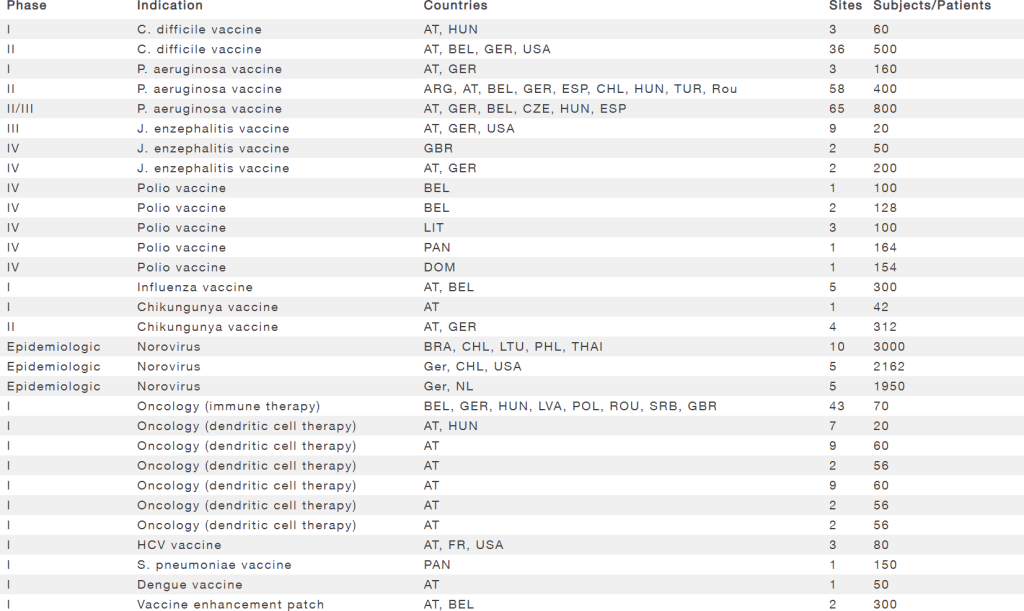
Experience in vaccine development
| Phase | Indication | Countries | Sites | Subjects/Patients |
|---|---|---|---|---|
| I | C. difficile vaccine | AT, HUN | 3 | 60 |
| II | C. difficile vaccine | AT, BEL, GER, USA | 36 | 500 |
| I | P. aeruginosa vaccine | AT, GER | 3 | 160 |
| II | P. aeruginosa vaccine | ARG, AT, BEL, GER, ESP, CHL, HUN, TUR, Rou | 58 | 400 |
| II/III | P. aeruginosa vaccine | AT, GER, BEL, CZE, HUN, ESP | 65 | 800 |
| III | J. enzephalitis vaccine | AT, GER, USA | 9 | 20 |
| IV | J. enzephalitis vaccine | GBR | 2 | 50 |
| IV | J. enzephalitis vaccine | AT, GER | 2 | 200 |
| IV | Polio vaccine | BEL | 1 | 100 |
| IV | Polio vaccine | BEL | 2 | 128 |
| IV | Polio vaccine | LIT | 3 | 100 |
| IV | Polio vaccine | PAN | 1 | 164 |
| IV | Polio vaccine | DOM | 1 | 154 |
| I | Influenza vaccine | AT, BEL | 5 | 300 |
| I | Chikungunya vaccine | AT | 1 | 42 |
| II | Chikungunya vaccine | AT, GER | 4 | 312 |
| Epidemiologic | Norovirus | BRA, CHL, LTU, PHL, THAI | 10 | 3000 |
| Epidemiologic | Norovirus | Ger, CHL, USA | 5 | 2162 |
| Epidemiologic | Norovirus | Ger, NL | 5 | 1950 |
| I | Oncology (immune therapy) | BEL, GER, HUN, LVA, POL, ROU, SRB, GBR | 43 | 70 |
| I | Oncology (dendritic cell therapy) | AT, HUN | 7 | 20 |
| I | Oncology (dendritic cell therapy) | AT | 9 | 60 |
| I | Oncology (dendritic cell therapy) | AT | 2 | 56 |
| I | Oncology (dendritic cell therapy) | AT | 9 | 60 |
| I | Oncology (dendritic cell therapy) | AT | 2 | 56 |
| I | Oncology (dendritic cell therapy) | AT | 2 | 56 |
| I | HCV vaccine | AT, FR, USA | 3 | 80 |
| I | S. pneumoniae vaccine | PAN | 1 | 150 |
| I | Dengue vaccine | AT | 1 | 50 |
| I | Vaccine enhancement patch | AT, BEL | 2 | 300 |

Our method
We understand the challenges that you face every day…because we have been there.
After analyzing a situation, we will help you to identify the optimal method of creating value for your products and technology on a scientific and on a business level – which can be very often a challenging task for small to medium sized companies due to a lack of resources or funding.
We operate from an entrepreneur’s perspective: one shot to get the alignment of business right. We earned credibility with customers and added momentum to investors and management.
We have observed that in today’s marketplace, good ideas, good technologies, promising compounds and good products have their highest value when they are able to find their niche quickly. This can be done via an efficient, cost- effective, and value-realizing route to market.
Based on the analysis, priorities are established and applied in the real world – we do not just talk and write, we will put our hands to the wheel.
Predicting rain doesn’t count, building arks does
Warren Buffet

Our value
We focus on people and on process, to promote business operations which are supported by the total resources of the organization – the basis for achievement and success.
Nur überlegen macht überlegen
Eduard Brauer

Why work with Finom Management Group?
We are PERSONAL: Our ideal size gives you direct access to decision makers for prompt responses and short turnaround times.
We are FLEXIBLE: the scope to offer you the full suite of clinical development services, and yet the flexibility to select the combination of expertise you need, fully tailored to your requirements.
We are RELIABLE: we are privately-owned with no third-party interests. You get an equal partner who shares your goals.
We generate VALUE: Our size and structure allows us to implement creative and innovative processes. This translates into significant cost savings without any compromises on quality.
QUALITY: Is a must. We use intelligence data from past successes and failures to deliver compliance and result-oriented trials.

Contact
Please contact us at:
Dr. Klaus Fischer
Président directeur général
phone: +43 676 765 2473
klaus.fischer@finommanagement.com

